One of the major differences between
laboratory organic reactions (which generally take place free in
solution) and biological organic reactions (which generally take place
within the very specific, ordered environment of an enzyme) involves the
concepts of stereoselectivity and stereospecificity. In a stereoselective reaction, one stereoisomer is formed preferentially over other possible stereoisomers:
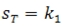
In section 14.1, we will learn about a reaction type in which a water molecule is ‘added’ to a double bond.
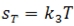
In many cases, this reaction results in
the formation of one, or possibly two new stereocenters, depending on
the symmetry of the starting alkene double bond. Nonenzymatic
laboratory reactions of this type generally are not stereospecific –
that is, they result in the formation of mixtures of different
stereoisomers. In contrast, a crucial aspect of enzyme-catalyzed
biological chemistry is that reactions are almost always highly
stereoselective, meaning that they result in the formation of only one
specific stereoisomer. For example, this water addition reaction occurs
as part of the oxidation of fatty acids:
Enzymatic reactions are also highly stereospecific:
this means that an enzyme ‘recognizes’ the stereochemistry of a
substrate molecule and only catalyzes its reaction if the substrate
stereochemistry is correct.
The enzyme that catalyzes the alkylation of (S)-glycerol phosphate, for example, will not work at all with (R)-glycerol phosphate.

When we begin our study of how organic
reactions are catalyzed by enzymes, the reasons for their remarkable
stereoselectivity and stereospecificity will become apparent.
After reading the story about
thalidomide at the beginning of our discussion of stereochemistry, you
have a good appreciation for the importance of stereoisomerism in drug
development. It is much safer and more effective if a drug can be
provided in stereochemically pure form, without the presence of other
ineffective (and possibly dangerous) enantiomers or diastereomers. Drug
that are obtained from nature are generally in stereochemically pure
form to begin with, because they are synthesized in a living organism by
a series of enzymatic reactions. Penicillin, with its three
stereocenters and 8 possible stereoisomers, is a good example: as a
product synthesized by specific enzymes in penicillum mold, it exists as a single stereoisomer.
Other chiral drugs must be synthesized
by humans in the laboratory, where it is much more difficult to produce a
single stereoisomer. Medicinal chemists are working very hard to
develop new laboratory synthetic techniques which will allow them to
better control the stereochemical outcome of their reactions. We will
see one example of such a technique in section 16.10B. In addition,
many researchers are trying to figure out the enzymatic process by which
living things produce useful chiral molecules, so that the enzymes
involved may be put to use as in vitro synthetic tools. This is important because, while it may be easy to obtain large quantities of penicillum mold, many other useful chiral compounds come from species that are difficult, or even impossible, to grow in the lab.
why the reaction of (R) - glycerol Phospate no reaction so that the formation of products please explain?
BalasHapusIn section 14.1, we will learn about a reaction type in which a water molecule is ‘added’ to a double bond.
BalasHapuswhy in this reaction there is 2 new stereocenter?
please your information haviz thank