A cyclic amide that is the nitrogen analog of a
lactone.
For example, a γ-aminobutyric acid readily forms γ-butyrolactam (also
known as 2-pyrrolidinone) upon heating, as in the reaction below. The
tautomeric
enol form of a lactam is known as a
lactim.

The
δ-amino acids similarly form δ (six-membered-ring) lactams upon
heating, but larger- and smaller-ring lactams must be made by indirect
methods.
Several lactams are of considerable industrial
importance. 2-Pyrrolidinone and 1-methyl-2-pyrrolidinone are made by
heating γ-butyrolactone with
ammonia and
methylamine, respectively. They are useful specialty solvents. Vinylation of 2-pyrrolidinone with
acetylene gives 1-vinyl-2-pyrrolidinone, which is
polymerized to a substance commonly used in
aerosol hair
sprays.
The ß-lactam
antibiotics comprise two groups of clinically important therapeutic agents, the
penicillins
and the cephalosporins. In both cases they contain a four-membered or
ß-lactam ring which has its nitrogen atom and a carbon atom in common
with another ring. Such substances are derived commercially from
fermentation processes, followed usually by chemical manipulation of the functional groups.
See also Amino acids; Biochemical engineering; Lactone.
any intramolecular cyclic amide produced by the formal removal of a
molecule of water between the amino and the carboxyl groups of an amino
acid, other than an
α-amino acid. A prefix (as in
β-lactam)
may be used to designate the position of the amino group in the parent
compound. A lactam commonly exists in tautomeric equilibrium with its
corresponding
lactim.
Lactones, lactams, lactims, and analogues
Compounds that may be considered as derived from a hydroxy catboxylic
acid or amino carboxylic acid by loss of water intramolecularly are
called generically "lactones" or "lactams",
respectively. Tautomeric forms of lactams are called "lactims". In these
recommendations, such compounds are preferably named as heterocycles
although names that may be considered to be derived from
the corresponding hydroxy or amino acid are also given.
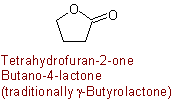
R-5.7.5.1 Lactones.
Inrtamolecular esters of hydroxy carboxylic acids are "lactones" and are
named as heterocycles or by substituting "-olactone"
for the "-ic acid" ending of a trivial name of a hydroxy acid, or
"-lactone" for the "-ic acid" ending of a systematic "-oic acid" name
for the nonhydroxylated parent acid, and inserting a locant
designating the position of the hydroxy group between the "o" and
"lactone"

Example to R-5.7.5.1
Heterocycles in which one or more (but not all) rings of a polycyclic
ring system are lactones are named bu adding the suffix "-carbolactone"
(denoting a cyclic
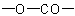
group) to the name
of the ring system left after the
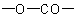
residue is replaced by two hydrogen atoms, preceded by a pair of
locants indicating the points of attachment of the carbonyl group and
the oxygen
atom of the lactone, respectively; the locant for the carbonyl group is
cited first, and, if there is a choice, is the lower locant. Multiplying
prefixes and pairs of locants separated by a colon
denote the precence of two or more lactone rings.
Examples to R-5.7.5.1
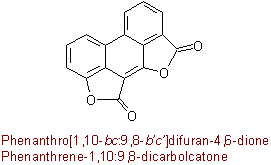
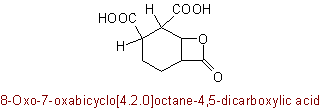
(R-2.4.2.1)
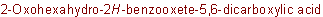
(R-2.4.4.1)
R-5.7.5.2 Sultones.
Intramolecular esters of hydroxy sulfonic acids are called "sultones"
and are named as heterocycles or by citing the term "sultone" denoting
the cyclic
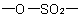
group after the name of the appropriate parent hydride preceded by a
pair of locants describing the points of attachment of the sulfonyl
group and oxygen atom, respectively; the
locant for the sulfonyl group is cited first and, if there is a choice,
is the lower locant. Multiplying prefixes and pairs of locants separated
by a colon are used to indicate two or more sultone
rings.
Examples to R-5.7.5.2
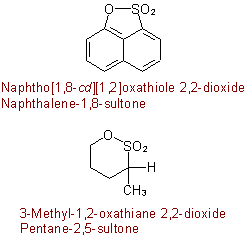
R-5.7.5.3 Lactams and lactims. Nitrogen analogues of lactones having the group
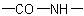
as part of a ring or ring system are called generically "lactams" and
their tautomers,
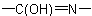
, are "lactims". These compounds are named as heterocyclic compounds or in accordance with
R-5.7.5.1 using "-lactam" or "-lactim", respectively, in place
of "-lactone".
Names such as "propiolactam" and "butyrolactim" are not included in these recommendations.
Examples to R-5.7.5.3
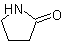 Tetrahydropyrrol-2-one
Tetrahydropyrrol-2-one
Pyrrolidin-2-one (2-Pyrrolidone )
)
Butano-4-lactam
R-5.7.5.4 Sultams. Nitrogen analogues of sultones having the group
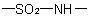
as a part of a ring are named as heterocycles or in accordance with
R-5.7.5.2
using "sultam" in place of "sultone". The locant for the point of
attachment of the sulfonyl group is cited first and, where there is a
choice, has preference over the imino group
for lower locant.
Examples to R-5.7.5.4
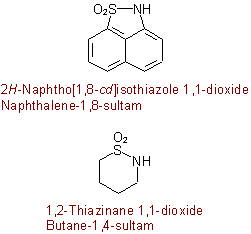



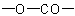 group) to the name
of the ring system left after the
group) to the name
of the ring system left after the 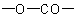 residue is replaced by two hydrogen atoms, preceded by a pair of
locants indicating the points of attachment of the carbonyl group and
the oxygen
atom of the lactone, respectively; the locant for the carbonyl group is
cited first, and, if there is a choice, is the lower locant. Multiplying
prefixes and pairs of locants separated by a colon
denote the precence of two or more lactone rings.
residue is replaced by two hydrogen atoms, preceded by a pair of
locants indicating the points of attachment of the carbonyl group and
the oxygen
atom of the lactone, respectively; the locant for the carbonyl group is
cited first, and, if there is a choice, is the lower locant. Multiplying
prefixes and pairs of locants separated by a colon
denote the precence of two or more lactone rings.


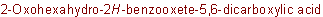
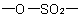 group after the name of the appropriate parent hydride preceded by a
pair of locants describing the points of attachment of the sulfonyl
group and oxygen atom, respectively; the
locant for the sulfonyl group is cited first and, if there is a choice,
is the lower locant. Multiplying prefixes and pairs of locants separated
by a colon are used to indicate two or more sultone
rings.
group after the name of the appropriate parent hydride preceded by a
pair of locants describing the points of attachment of the sulfonyl
group and oxygen atom, respectively; the
locant for the sulfonyl group is cited first and, if there is a choice,
is the lower locant. Multiplying prefixes and pairs of locants separated
by a colon are used to indicate two or more sultone
rings.

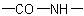 as part of a ring or ring system are called generically "lactams" and
their tautomers,
as part of a ring or ring system are called generically "lactams" and
their tautomers, 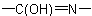 , are "lactims". These compounds are named as heterocyclic compounds or in accordance with
, are "lactims". These compounds are named as heterocyclic compounds or in accordance with 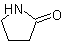 Tetrahydropyrrol-2-one
Tetrahydropyrrol-2-one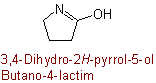
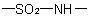 as a part of a ring are named as heterocycles or in accordance with
as a part of a ring are named as heterocycles or in accordance with

Tidak ada komentar:
Posting Komentar