In low doses (an average cigarette yields about 1 mg of absorbed nicotine), the substance acts as a stimulant in mammals, while high amounts (30–60 mg) can be fatal. This stimulant effect is the main factor responsible for the dependence-forming properties of tobacco smoking. According to the American Heart Association, nicotine addiction has historically been one of the hardest addictions to break, while the pharmacological and behavioral characteristics that determine tobacco addiction are similar to those determining addiction to heroin and cocaine. The nicotine content of popular American-brand cigarettes has slowly increased over the years, and one study found that there was an average increase of 1.6% per year between the years of 1998 and 2005. This was found for all major market categories of cigarettes.
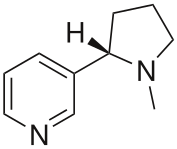
Nicotine is a hygroscopic, oily liquid that is miscible with water in its base form. As a nitrogenous base, nicotine forms salts with acids that are usually solid and water soluble. Nicotine easily penetrates the skin. As shown by the physical data, free base nicotine will burn at a temperature below its boiling point, and its vapors will combust at 308 K (35 °C; 95 °F) in air despite a low vapor pressure. Because of this, most of the nicotine is burned when a cigarette is smoked; however, enough is inhaled to cause pharmacological effects.
The biosynthetic pathway of nicotine involves a coupling reaction between the two cyclic structures that compose nicotine. Metabolic studies show that the pyridine ring of nicotine is derived from nicotinic acid while the pyrrolidone is derived from N-methyl-Δ1-pyrrollidium cation.[18][19] Biosynthesis of the two component structures proceeds via two independent syntheses, the NAD pathway for nicotinic acid and the tropane pathway for N-methyl-Δ1-pyrrollidium cation.
The NAD pathway in the genus nicotiana begins with the oxidation of aspartic acid into α-imino succinate by aspartate oxidase (AO). This is followed by a condensation with glyceraldehyde-3-phosphate and a cyclization catalyzed by quinolinate synthase (QS) to give quinolinic acid. Quinolinic acid then reacts with phosphoriboxyl pyrophosphate catalyzed by quinolinic acid phosphoribosyl transferase (QPT) to form nicotinic acid mononucleotide (NaMN). The reaction now proceeds via the NAD salvage cycle to produce nicotinic acid via the conversion of nicotinamide by the enzyme nicotinamidase.
The N-methyl-Δ1-pyrrollidium cation used in the synthesis of nicotine is an intermediate in the synthesis of tropane-derived alkaloids. Biosynthesis begins with decarboxylation of ornithine by ornithine decarboxylase (ODC) to produce putrescine. Putrescine is then converted into N-methyl putrescine via methylation by SAM catalyzed by putrescine N-methyltransferase (PMT). N-methylputrescine then undergoes deamination into 4-methylaminobutanal by the N-methylputrescine oxidase (MPO) enzyme, 4-methylaminobutanal then spontaneously cyclize into N-methyl-Δ1-pyrrollidium cation.
The final step in the synthesis of nicotine is the coupling between N-methyl-Δ1-pyrrollidium cation and nicotinic acid. Although studies conclude some form of coupling between the two component structures, the definite process and mechanism remains undetermined. The current agreed theory involves the conversion of nicotinic acid into 2,5-dihydropyridine through 3,6-dihydronicotinic acid. The 2,5-dihydropyridine intermediate would then react with N-methyl-Δ1-pyrrollidium cation to form enantiomerically pure (–)-nicotine.

In consumption it most commonly appears in the forms of smoking, chewing, snuffing, or dipping tobacco. Tobacco had long been in use as an entheogen in the Americas, but upon the arrival of Europeans in North America, it quickly became popularized as a trade item and a widely-abused drug. This popularization led to the development of the southern economy of the United States until it gave way to cotton. Following the American Civil War, a change in demand and a change in labor force allowed for the development of the cigarette. This new product quickly led to the growth of tobacco companies. There are more than 70 species of tobacco in the plant genus Nicotiana. The word nicotiana (as well as nicotine) is in honor of Jean Nicot, French ambassador to Portugal, who in 1559 sent it as a medicine to the court of Catherine de Medici.
Because of the powerfully addictive properties of nicotine, tolerance and dependence develop. Absorption quantity, frequency, and speed of tobacco consumption are believed to be directly related to biological strength of nicotine dependence, addiction, and tolerance. The usage of tobacco is an activity that is practiced by some 1.1 billion people, and up to 1/3 of the adult population.The World Health Organization(WHO) reports it to be the leading preventable cause of death worldwide and estimates that it currently causes 5.4 million deaths per year.Rates of smoking have leveled off or declined in developed countries, but continue to rise in developing countries.
Tobacco is cultivated similarly to other agricultural products. Seeds are sown in cold frames or hotbeds to prevent attacks from insects, and then transplanted into the fields. Tobacco is an annual crop, which is usually harvested mechanically or by hand. After harvest, tobacco is stored for curing, which allows for the slow oxidation and degradation of carotenoids. This allows for the agricultural product to take on properties that are usually attributed to the "smoothness" of the smoke. Following this, tobacco is packed into its various forms of consumption, which include smoking, chewing, snuffing, and so on. Most cigarettes incorporate flue-cured tobacco, which produces a milder, more inhalable smoke. Use of low-pH, inhalable, flue-cured tobacco is one of the principal reasons smoking causes lung cancer and other diseases association with smoke inhalation.

i've some problem
BalasHapuswhat caused the tobacco is dangerous when in his journal in saying that the nicotine contained in tobacco beneficial to health? please confirm that