In chemistry, the term amide has
several meanings. It may refer to a particular inorganic anion, it may
refer to a functional group found in organic compounds, or to compounds
that contain this functional group.
The amide anion is the conjugate base of ammonia,
NH2-. It is an extremely strong base, due to the extreme weakness of
ammonia as a Bronsted acid.
Amides are the members of a group of chemical
compounds containing nitrogen. Specifically, an amide is a derivative of
a carboxylic acid in which the hydroxyl group has been replaced by an
amine or ammonia.
Compounds in which a hydrogen atom on nitrogen from
ammonia or an amine is replaced by a metal cation are also known as
amides or azanides. The amide functional group is:

Synthesis and breakdown
Amides are commonly formed from the reaction of a carboxylic acids with an amine:

This is the reaction that forms peptide bonds between
amino acids. These amides can participate in hydrogen bonding as
hydrogen bond acceptors and donors, but do not ionize in aqueous
solution, whereas their parent acids and amines are almost completely
ionized in solution at neutral pH.
Amide formation plays a role in the synthesis of some
condensation polymers, such as nylon. Their breakdown is possible via
amide hydrolysis.
Amide linkages
An amide linkage is kinetically stable to hydrolysis.
Amide linkages in a biochemical context are called peptide linkages.
Amide linkages constitute a defining molecular feature of proteins, the
secondary structure of which is due in part to the hydrogen bonding
abilities of amides.
Derivatives
Sulfonamides are analogs of amides in which the atom double bonded to oxygen is sulfur rather than carbon.
Naming
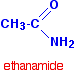
- Example: CH3CONH2 is named acetamide or ethanamide
- Other examples: propan-1-amide, N,N-dimethylpropanamide
The most commonly discussed amide is ethanamide, CH3CONH2 (old name: acetamide).
| HCONH2 | methanamide |
| CH3CONH2 | ethanamide |
| CH3CH2CONH2 | propanamide |
If the chain was branched, the carbon in the -CONH2 group counts as the number 1 carbon atom. For example:
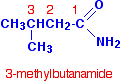
Tidak ada komentar:
Posting Komentar